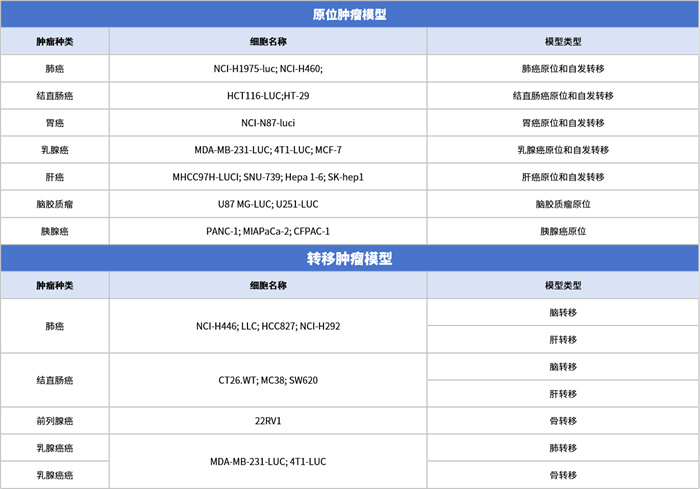
New Thoughts on Tumor Animal Models
As cancer is gradually recognized by humans, more and more tumor models are being used, such as subcutaneous xenograft and in situ xenograft models, aimed at simulating tumor growth and disease development, providing reference for treatment plans, and saving the health and lives of more patients.
However, with the rapid development of biomedical technology, people's understanding of the human body has gradually become clearer. For tumors, we have found that it is not only necessary to understand the tumor itself, but also factors such as the tumor microenvironment and cell metastasis preferences are increasingly valued.Meanwhile, an increasing amount of clinical data shows that the vast majority of cancer patients die from tumor metastasis.
New understanding of in situ tumors and tumor metastasis
In recent years, there have been increasing reports on research related to in situ metastatic tumor models, with over 4800 articles published in PubMed in the past five years using the keyword "exotic tumor model" for retrieval. These studies involve the microenvironment of in situ tumors, invasion of surrounding tissues, tumor metastasis, and interventional therapy.
In clinical practice, research on therapeutic targets and drugs related to tumor metastasis is becoming increasingly important and needed.
From the perspective of drug metabolism distribution, studies in situ tumor models are closer to the pharmacological efficacy research results under actual drug metabolism distribution conditions in clinical stages, and have more guiding significance for clinical trial design in terms of dosage and administration frequency settings. These characteristics of in situ metastatic tumor models cannot be replaced by subcutaneous tumor models.
KCI•KMQ has rich experience in developing in situ and metastatic models in the field of tumors, with high surgical success rates and low mortality rates.
At present, human/mouse derived subcutaneous tumor models, in situ tumor models, and metastatic tumor models have been successfully established, covering common tumor types and facilitating the development of new cancer drugs.
Data Display
Bone metastasis often occurs in breast cancer and prostate cancer, resulting in destruction of bone tissue. Bone metastasis models of breast cancer or prostate cancer can be established by injecting tumor cells into the femoral medullary cavity. Pathological staining in Figure 1a shows that the blue arrow represents invasive growth of tumor tissue and bone tissue destruction in the bone marrow cavity; The pink arrow represents cortical bone resorption in the backbone, caused by invasive tumor growth. The yellow arrow represents the invasive growth of tumor cells and the dissolution of bone tissue. The asterisk represents the bone marrow cavity, tumor tissue, and necrosis, with no normal bone marrow tissue.
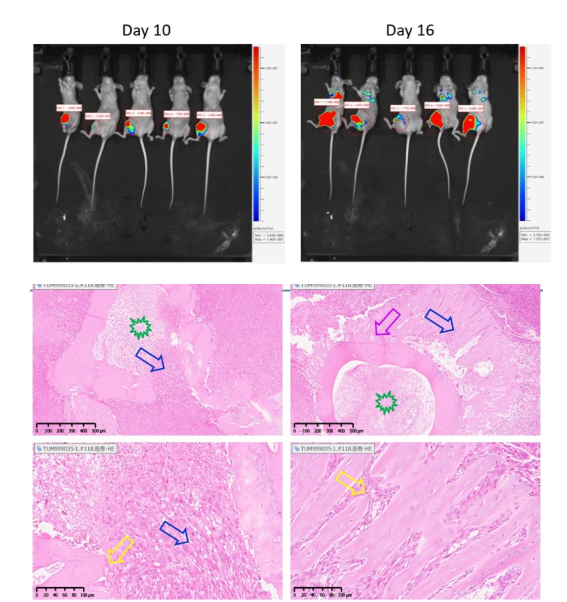
An in situ model of lung cancer can be established by injecting LLC tumor cells into the lungs of mice. As shown in Figure 2a, tumors can infiltrate and grow around the lungs and blood vessels (left figure); The right figure in Figure 2a shows the tumor infiltrating the lungs and metastasizing to the mediastinum. Figure 2b shows the tumor microenvironment and immune infiltration.
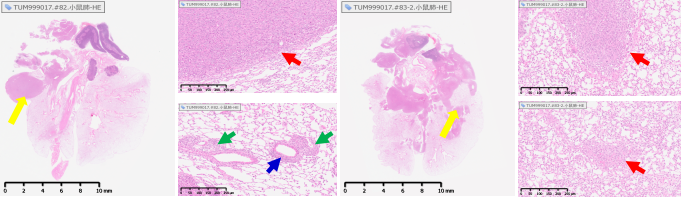
Figure 2 (a) LLC lung cancer in situ model
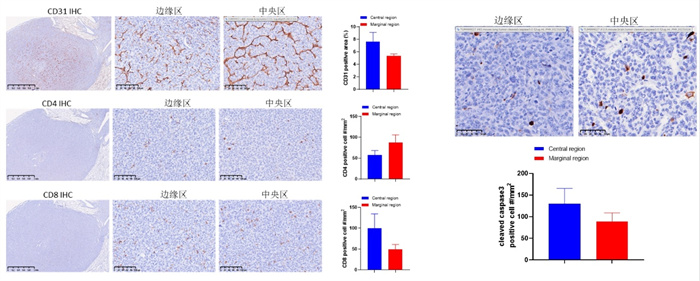
Figure 2 (b) LLC lung cancer in situ model microenvironment and immune infiltration
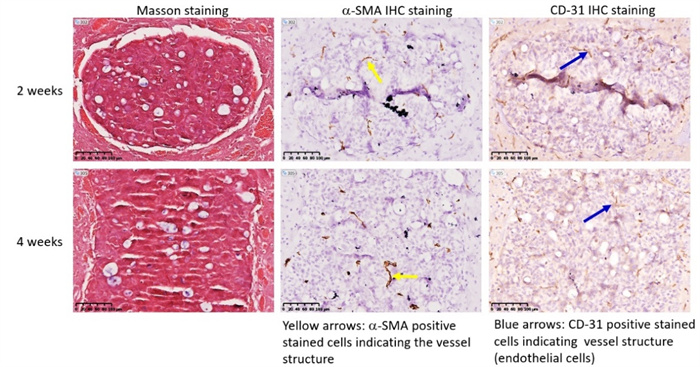
Lung cancer often undergoes brain metastasis. Using HCC827 lung cancer cell line for intracranial inoculation can establish a lung cancer brain metastasis model (Figure 3a). Through intratumoral IHC staining, neovascularization (SMA positive, CD-31 positive) can be observed. Refer to Figure 3b.
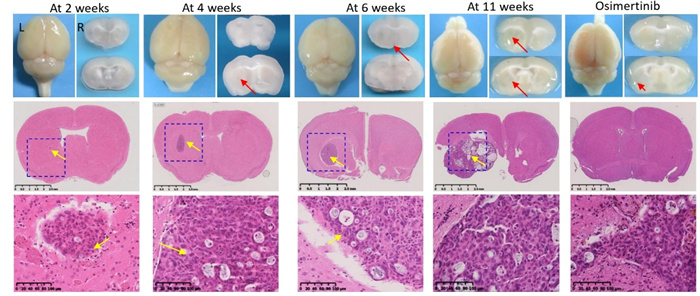
Figure 3 (a) HCC827 lung cancer brain metastasis model
In situ liver cancer models can be constructed by using the liver cancer cell line MHCC97H-LUCI for liver in situ inoculation. Pathology shows tumors at the site of intrahepatic inoculation and nodules formed at the site of metastasis.
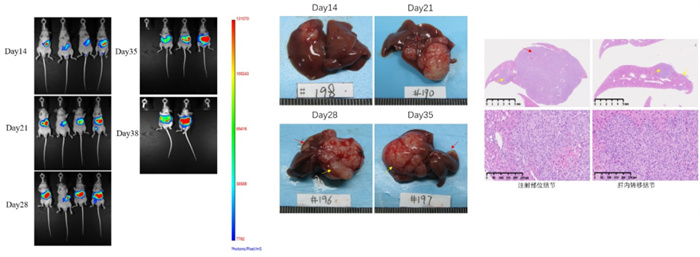
Figure 4. In situ model of MHCC97H-LUCI liver cancer
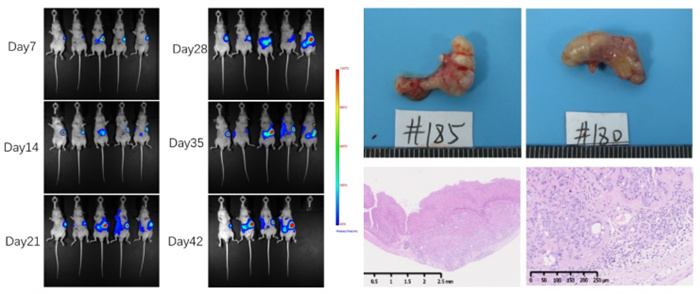
Figure 5. NCI-N87-LUCI Gastric Cancer In Situ Model
The in situ model of pancreatic cancer can be established by inoculating pancreatic cancer cell line PANC-1 in the pancreas (Fig. 6a). During the growth process of in situ tumors in pancreatic tissue, there is a significant proliferation of connective tissue, which separates tumor cells (A&B). FAP was expressed in pancreatic cancer cells and proliferative fibroblasts; At the same time, pancreatic acinar cells adjacent to the cancer highly express FAP, but normal pancreatic acinar cells far from the cancer tissue do not express FAP (C, D, E&F).
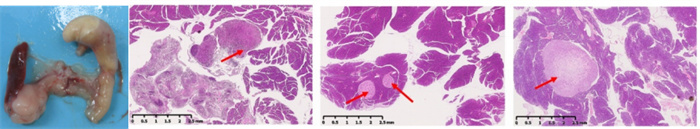
Figure 6 (a) PANC-1 in situ model of pancreatic cancer
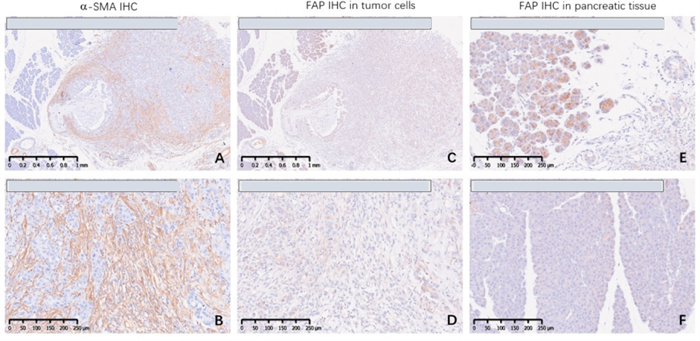
Figure 6 (b) Expression of SMA and FAP in PANC-1 pancreatic cancer in situ model
Inoculating human colorectal cancer HCT116-LUC cells into the cecum can establish an in situ model of colon cancer.

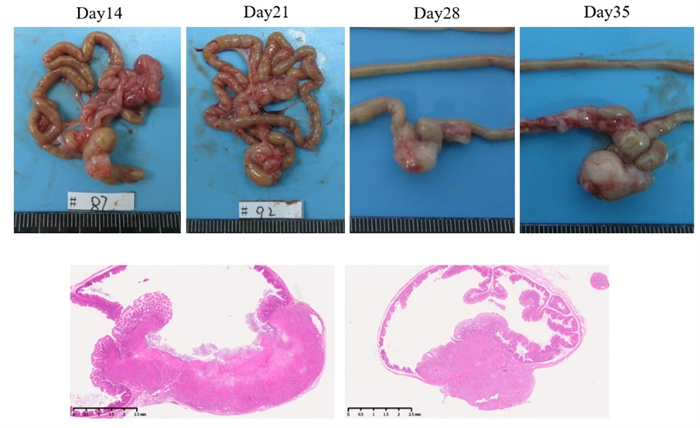
Figure 7. In situ model of HCT116-LUC colon cancer
In situ inoculation of mouse breast cancer 4T1-LUC cell line in mouse mammary gland can establish an in situ mammary gland model. Pathological results show metastasis of the tumor within the lungs and blood vessels.
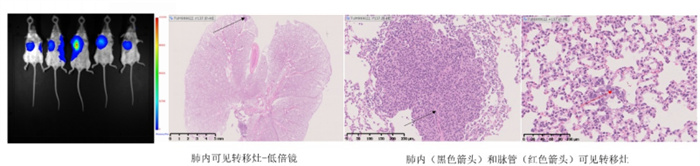
Figure 8. 4T1-LUC breast cancer in situ model
About KCI
KCI Biotech (Suzhou) Inc. and its wholly owned subsidiary Jiangsu KMQ Biotech Inc. are committed to providing comprehensive preclinical new drug research and development services to global pharmaceutical companies and research institutions, and establishing a professional and efficient drug research and development evaluation system. KCI·KMQ has two internationally advanced experimental animal facilities in Suzhou and Nantong, both of which have obtained international AAALAC certification, with a total area of over 18000 square meters; We have established a full range of experimental animal platforms including mice, hamsters, guinea pigs, rabbits, cats, dogs, pigs, ferrets, and non-human primates, over 400 animal models of tumors and non tumor diseases, ABSL-2/BSL-2 anti infective drug and vaccine evaluation experimental platforms, pathology, early toxicology, cell and molecular biology, pharmacokinetics, medical imaging, and other research service platforms.





