Regarding atopic dermatitis
Atopic dermatitis (AD), also known as atopic eczema, is a chronic, refractory, recurrent, itchy, and inflammatory skin disease.
The incidence rate of AD is on the rise all over the world. In China, the number of patients with atopic dermatitis is also increasing year by year. The WHO Burden of Disease Study shows that the number of AD patients worldwide is as high as 230 million, making it the skin disease with the highest disease burden among non fatal diseases.
In 2024, the Chinese Medical Association Dermatology Branch and other units jointly formed the "Expert Consensus on the Management of Drug Application for the Treatment of Atopic Dermatitis (2024 Edition)".
The consensus mainly recommends antihistamines, immunosuppressants, systemic steroids, biologics, and JAK inhibitors as routine treatment methods.
In the consensus, it is clear that TCS is currently the first-line treatment for AD. At the same time, it was proposed to regularly evaluate the skin adverse reactions of TCS, especially for patients who have been using potent TCS for a long time or TCS on thin and tender skin areas.For sensitive and weak skin areas, TCI is recommended as a treatment method.
In addition to hormone drugs, consensus also suggests that phosphodiesterase 4 (PDE-4) inhibitors can be used for all skin lesions, including those in thin and tender areas and folds, and can be initially used in combination with TCS.
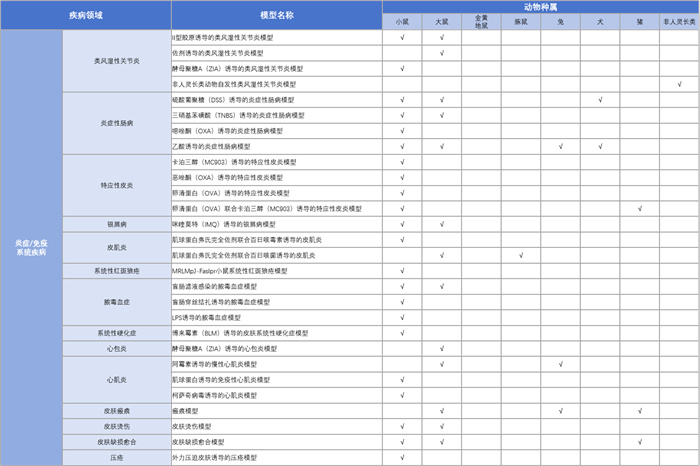
KCI Inflammation/Immune System Disease Model Platform
Data Display
Experimental animal: Mouse
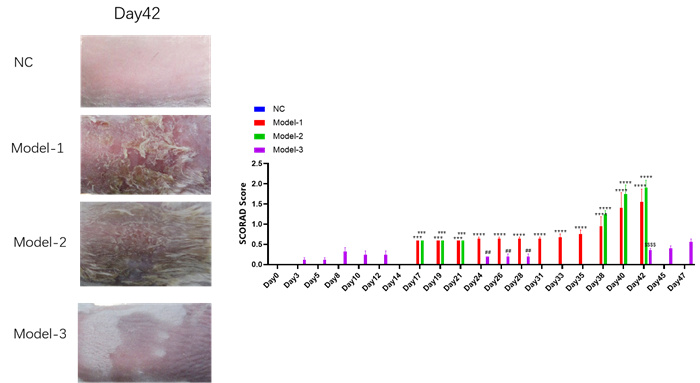
Experimental objective: To conduct pharmacological experiments on OVA induced mice, establish different models under conditions such as stimulation timeline, and determine the therapeutic effect of the test substance on atopic dermatitis.SCORAD score
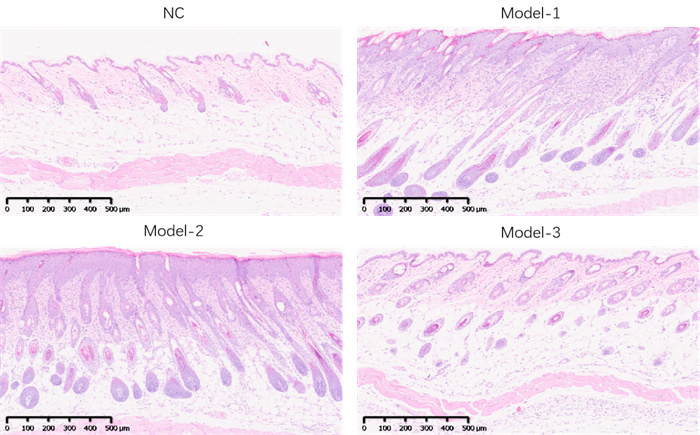
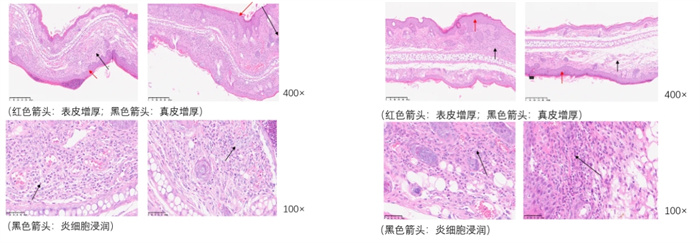
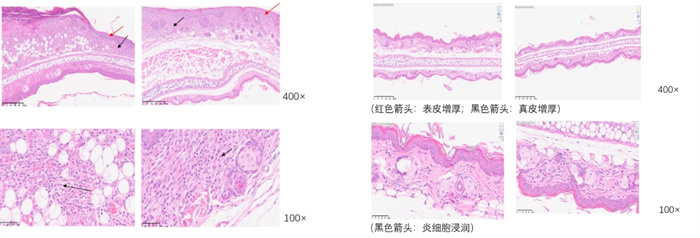
Skin thickness - HE staining
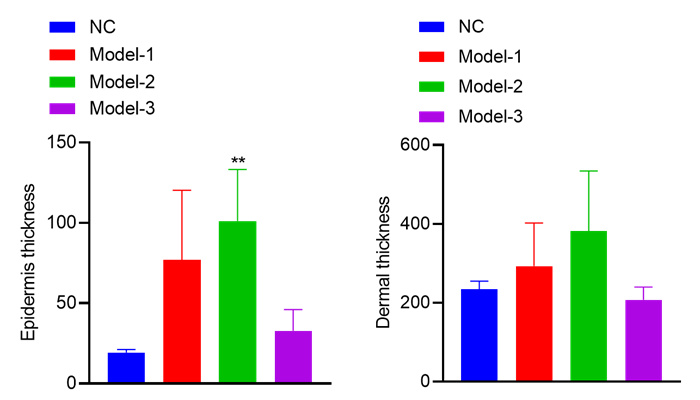
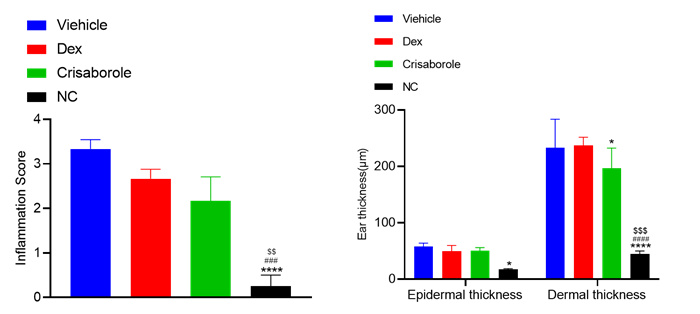
Thickness of genuine leather and epidermis
Inflammation score - HE staining
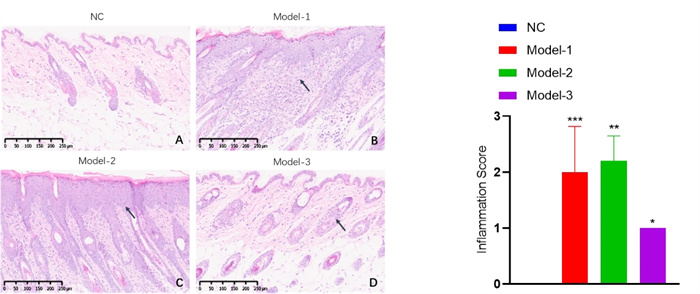
Mast cell scoring - toluidine blue staining
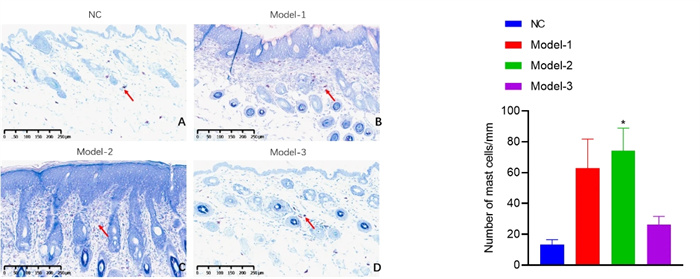
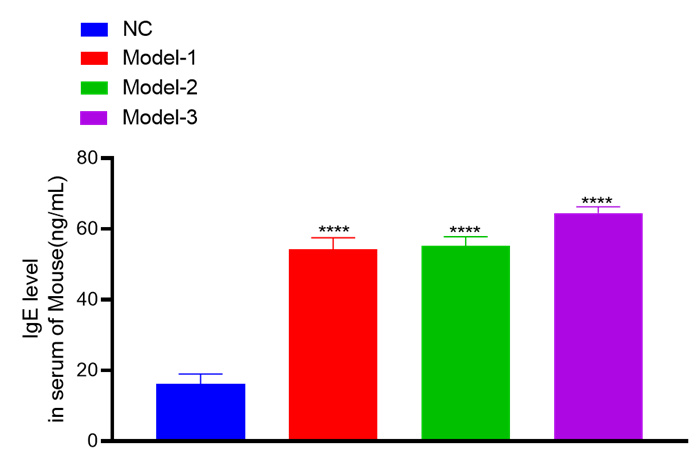
IgE level
Data Display
A mouse model of atopic dermatitis induced by oxazolone
Epigenetic observation
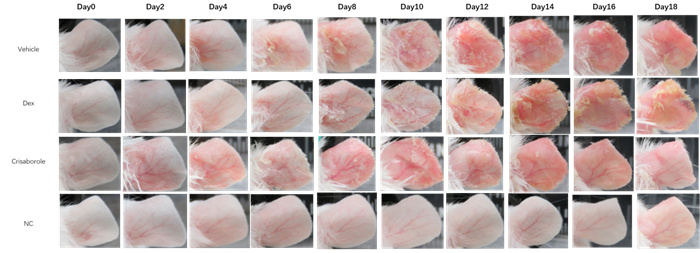
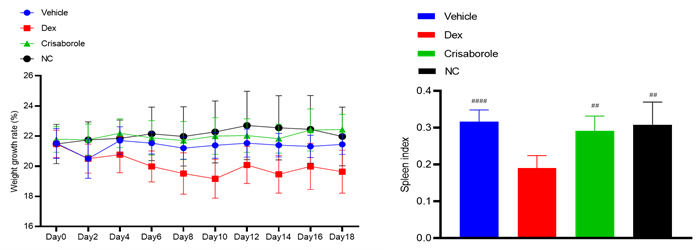
Weight changes & spleen index
SCORAD评分
IgE level

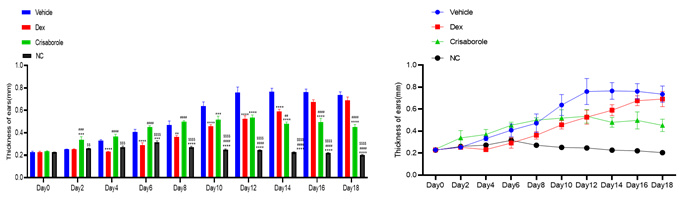
Skin thickness&inflammation score - HE staining
About KCI
KCI Biotech (Suzhou) Inc. and its wholly owned subsidiary Jiangsu KMQ Biotech Inc. are committed to providing comprehensive preclinical new drug research and development services to global pharmaceutical companies and research institutions, and establishing a professional and efficient drug research and development evaluation system. KCI·KMQ has two internationally advanced experimental animal facilities in Suzhou and Nantong, both of which have obtained international AAALAC certification, with a total area of over 18000 square meters; We have established a full range of experimental animal platforms including mice, hamsters, guinea pigs, rabbits, cats, dogs, pigs, ferrets, and non-human primates, over 400 animal models of tumors and non tumor diseases, ABSL-2/BSL-2 anti infective drug and vaccine evaluation experimental platforms, pathology, early toxicology, cell and molecular biology, pharmacokinetics, medical imaging, and other research service platforms.






