External preparations: mainly involve nano materials and hydrogels, which have become the research hotspot in recent years.
Biological agent: Ebglyss is a recently approved biological agent by the US FDA, which reduces skin inflammation and itching by targeting and inhibiting interleukin-13 (IL-13), a cytokine that plays an important role in the inflammatory process.Monoclonal antibody: On September 12, 2024, Conova's Class 1 new drug, Kangyueda (Sipuximab Injection), was officially approved by the National Medical Products Administration for the treatment of moderate to severe atopic dermatitis in adults.
Monoclonal antibody: Rocatinlimab is a novel OX40 monoclonal antibody, which is a molecule that regulates T cell activity. The drug has shown significant long-term effects in clinical trials, even maintaining skin improvement after patients stop taking it.JAK inhibitor: Upadacitinib is a Janus kinase (JAK) inhibitor that inhibits overactive immune responses by blocking the signaling pathways of multiple cytokines involved in inflammatory responses.
Peptide drug: '1104' is a novel peptide drug currently in preclinical research stage. Unlike other existing drugs, '1104' not only acts on the Th2 inflammatory pathway, but also affects various inflammatory factors associated with atopic dermatitis pathology.KCI Biological Inflammation/Immune System Disease Model Platform
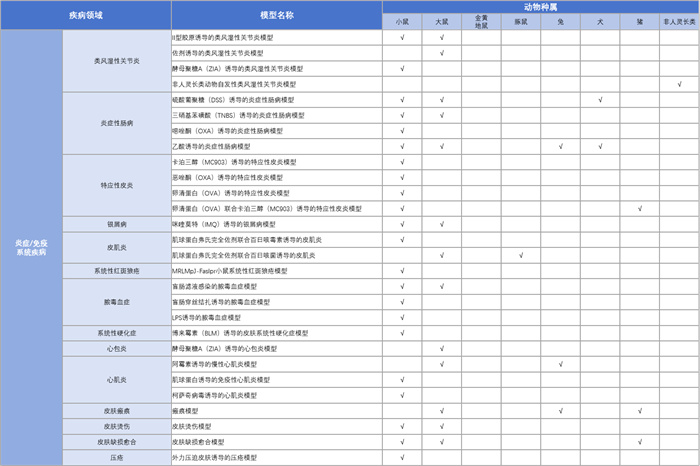
Data Display
Experimental animal: Mouse
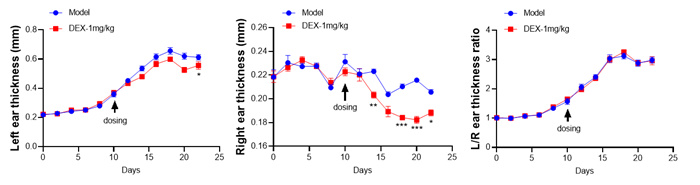
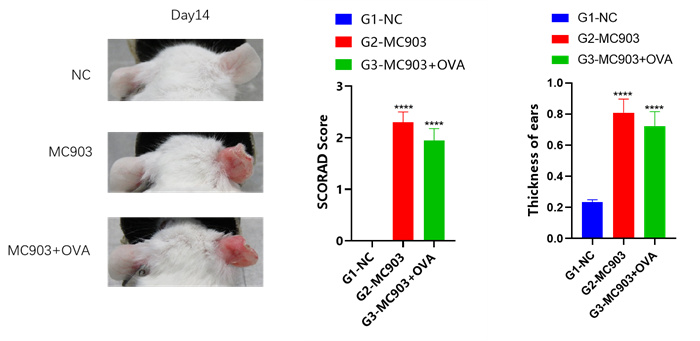
Experimental objective: To conduct efficacy tests on MC903 induced mice and determine the therapeutic effect of the test substance on atopic dermatitis.Clinical observation
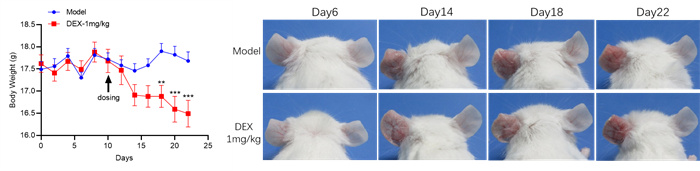
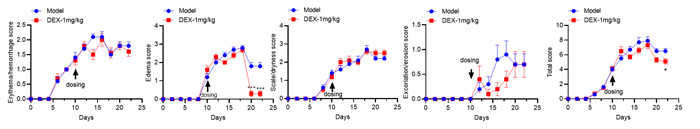
Ear & Spleen
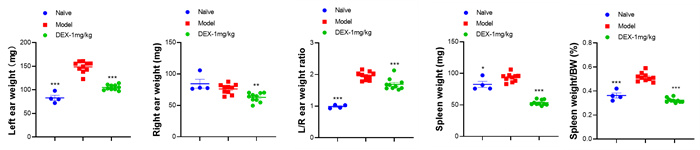
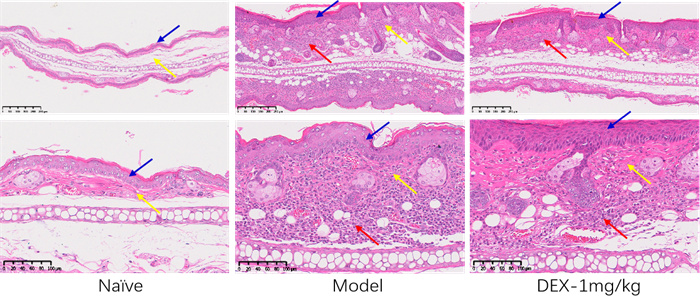
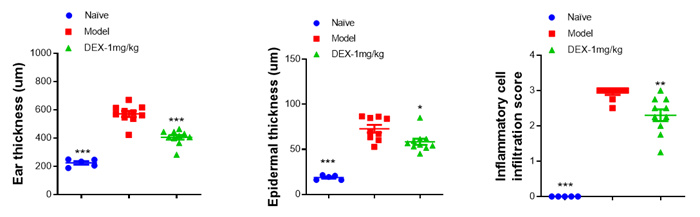
Data Display
Experimental animal: Mouse
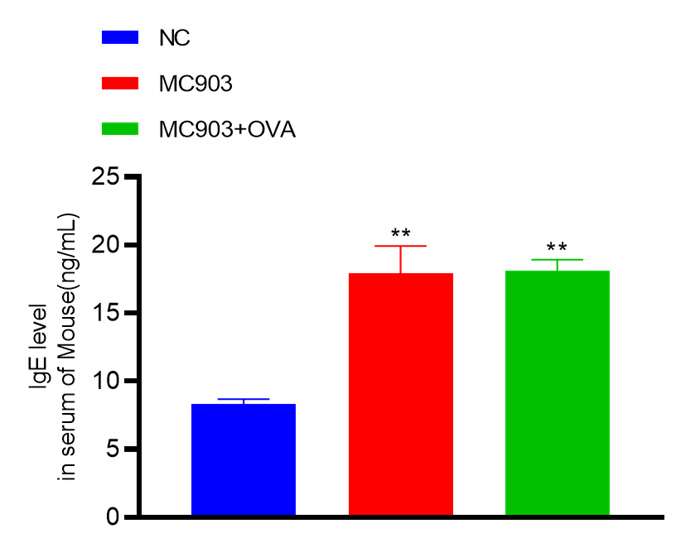
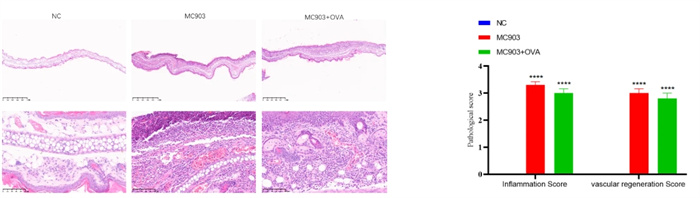
Experimental objective: To conduct pharmacological tests on mice induced by MC903 and MC903+OVA, respectively, to determine the therapeutic effect of the test substance on atopic dermatitis.Epigenetic observation
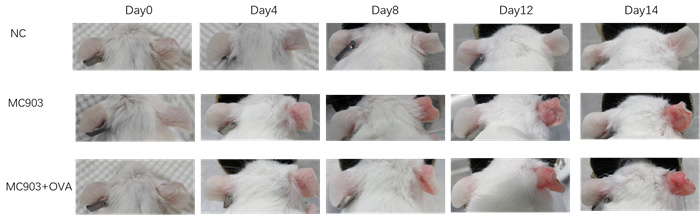
SCORAD score+ear thickness

IgE level
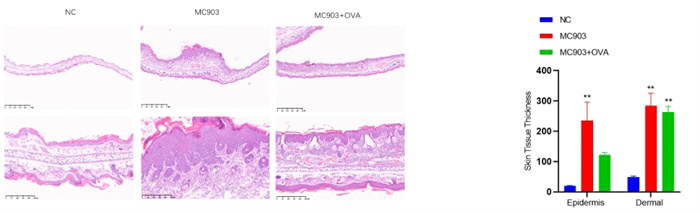
Inflammation&Vascular Regeneration Score - HE Staining
About KCI
KCI Biotech (Suzhou) Inc. and its wholly owned subsidiary Jiangsu KMQ Biotech Inc. are committed to providing comprehensive preclinical new drug research and development services to global pharmaceutical companies and research institutions, and establishing a professional and efficient drug research and development evaluation system. KCI·KMQ has two internationally advanced experimental animal facilities in Suzhou and Nantong, both of which have obtained international AAALAC certification, with a total area of over 18000 square meters; We have established a full range of experimental animal platforms including mice, hamsters, guinea pigs, rabbits, cats, dogs, pigs, ferrets, and non-human primates, over 400 animal models of tumors and non tumor diseases, ABSL-2/BSL-2 anti infective drug and vaccine evaluation experimental platforms, pathology, early toxicology, cell and molecular biology, pharmacokinetics, medical imaging, and other research service platforms.






