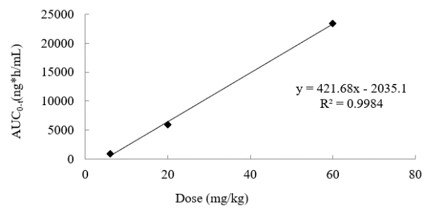
In vivo PK study
In in vivo pharmacokinetic (PK) experiments, the modes of administration include, but are not limited to intravenous, oral, administration, intraperitoneal injection,intramuscular injection, subcutaneous injection, ventricular administration, intrathecal injection, nasal administration, and tracheal administration. The sampling time point can be set at 24 hours or more according to the characteristics of the compound and the specific requirements of the project, to ensure a comprehensive evaluation of the dynamic changes of the drug in the body.
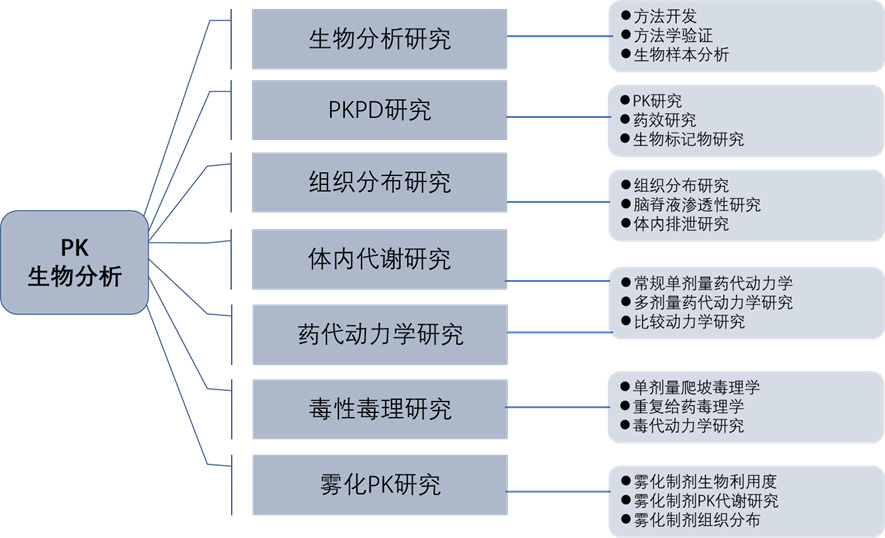
Our services
experiment | describe | Species and genus | result |
Conventional single dose pharmacokinetics | Single dose administration for each route of administration; | Mouse, rat, rabbit, dog, monkey | Medication time curve graph |
Multi dose pharmacokinetics | Single dose administration for each route of administration; | Mouse, rat, rabbit, dog, monkey | Medication time curve graph |
Comparative pharmacokinetics | Test formulation and reference formulation; | Mouse, rat, rabbit, dog, monkey | Medication time curve graph |