Definition and Overview:
Premature ovarian failure (POF) refers to the decline of ovarian function in women before the age of 40, accompanied by amenorrhea or menstrual disorders, high levels of gonadotropins (FSH), and low levels of estrogen. Its incidence rate accounts for about 1% of women of the same age.
clinical manifestation:
Patients may experience amenorrhea, infertility, and menopausal symptoms such as hot flashes, night sweats, and emotional fluctuations. Long term complications may include osteoporosis and cardiovascular disease.
Causes and Effects:
The etiology is complex, including autoimmune, genetic (such as X chromosome abnormalities, insufficient number of follicles at birth), iatrogenic damage, etc. Chemotherapy is one of the important triggers, especially alkylating agents such as cyclophosphamide, which can damage the ovaries and cause premature ovarian failure in young patients. Unless women with primary ovarian insufficiency receive estrogen therapy before the age of 51 (the average age of menopause), the risk of osteoporosis, dementia, Parkinson's disease, depression, and coronary artery disease will increase.
Clinical significance:
POF seriously affects fertility and quality of life. At present, hormone replacement therapy is mainly used for symptomatic relief, and new intervention methods are urgently needed to protect or restore ovarian function.
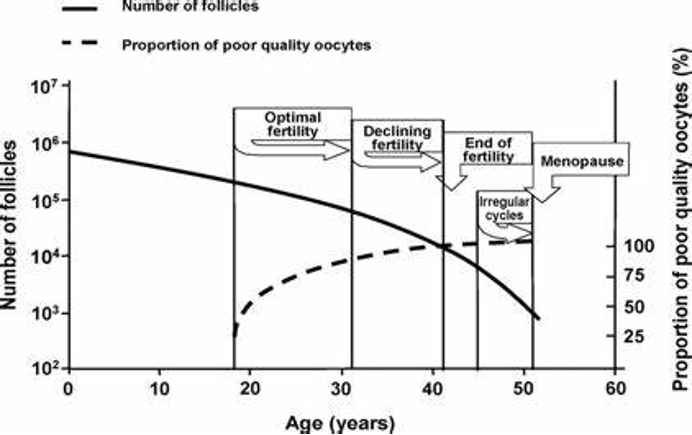
Ovarian function decline curve with age
02.Premature ovarian failure model
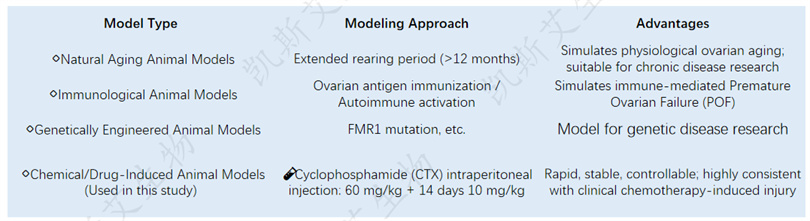
📌 Characteristics of this research model:
✅ Successfully induced ovarian atrophy, follicular depletion, dysregulation of the estrous cycle, and elevation of LH/FSH
✅ Pathological mechanism of POF induced by highly simulated clinical chemotherapy
✅ Applicable for the efficacy verification of ovarian protection and repair drugs (such as NAD+)
Characteristics of CTX induced POF model
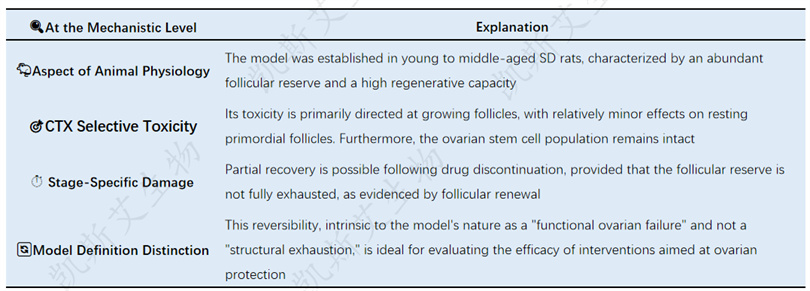
📌 Conclusion: This model is not an absolute ovarian termination model, but a "controllable functional inhibition" model, which is closer to the scene of early ovarian injury after chemotherapy.
Comparative Analysis Table of Animal Models and Human POF
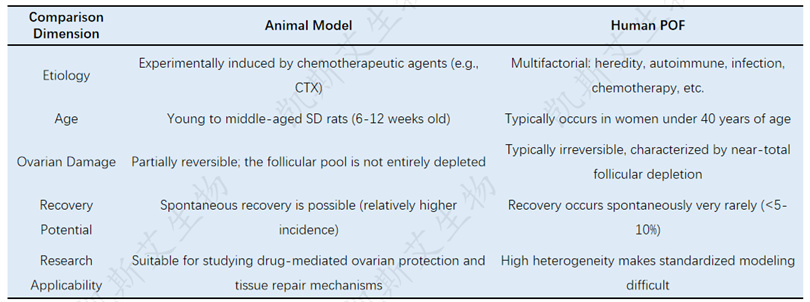
📌 Clinical implications: NAD+validated in this model is to 'delay and improve' ovarian function inhibition, rather than reverse absolute failure.
In the future, it may be applicable to:
✔ Ovarian protection in tumor treatment
✔ Adjuvant therapy for individuals with hormone contraindications
03.Treatment of premature ovarian failure
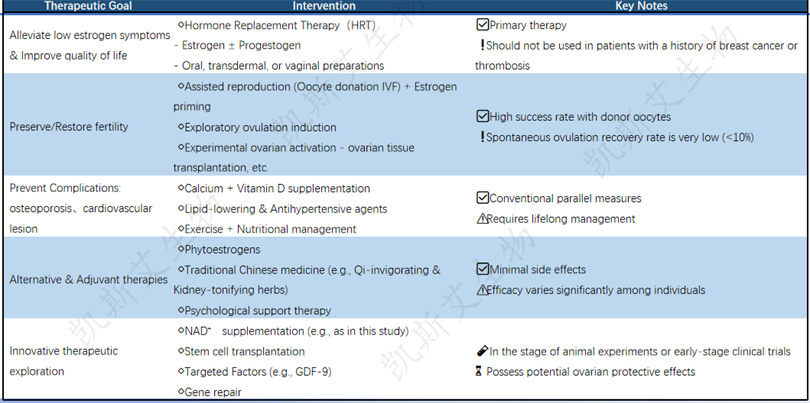
Summary: Currently, combined hormone therapy or hormone replacement+assisted reproduction is still the main treatment, lasting until the age of 51 (the average age of menopause). Non hormonal strategies such as NAD+may become an important complementary pathway for future POF interventions.
04.Protocol
• Objective of the test
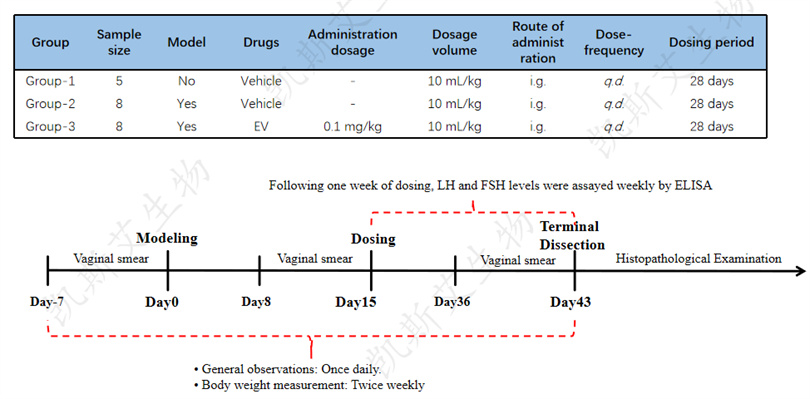
Explore the pharmacological effects of compound NAD+on cyclophosphamide induced premature ovarian failure in rats, and provide data support for subsequent experiments.
• Experimental animals
40 SD rats, female
• Modeling method
Cyclophosphamide (CTX) was injected intraperitoneally for the first time at a dose of 60 mg/kg, followed by daily injections of 10 mg/kg for 14 days, with a total modeling time of 15 days.
• testing indicators
--Internal: Daily observation (once a day);
--Weight (twice a week);
--Vaginal smear - time points: 7:00-8:00, before modeling (for 7 consecutive days), during modeling (for 7 consecutive days), and in the last week of administration (for 7 consecutive days), for a total of 21 days of testing;
--Hormone testing: serum follicle stimulating hormone (FSH) and luteinizing hormone (LH);
--Pathological examination of ovarian tissue: H& E
weight change
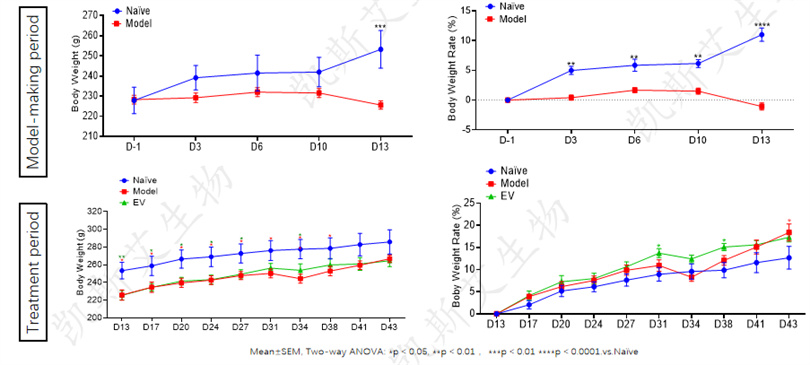
• During the CTX modeling period, the weight gain of the model group rats was significantly reduced, and by the 13th day of modeling, the average weight and weight gain rate were significantly lower than those of the normal group (p<0.001)
• After entering the treatment period, the weight of each treatment group gradually increased. The EV positive group had a weight recovery close to normal on the 38th day; The model control group did not approach recovery until the 41st day.
Emotional Cycle Statistics
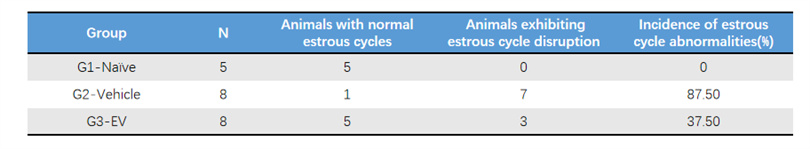
• The normal control rats had a regular estrous cycle with an abnormality rate of 0%, while the untreated model group had an abnormality rate as high as 87.5%, showing significant estrous cycle disorder.
• After 4 weeks of treatment, EVs significantly improved the regularity of the estrous cycle, indicating that EVs can effectively restore the estrous cycle rhythm of POF model animals.
Changes in serum follicle stimulating hormone (FSH) levels
FSH (Follicle Stimulating Hormone)
• Source: Secreted by the pituitary gland.
• Function: It is the "activation signal" of the ovary, mainly used to stimulate the growth and development of follicles in the ovary. Like a 'project manager', urging follicles to work.
• Meaning in the POI model: When ovarian function fails, follicles cannot develop normally and produce sufficient hormones, and the brain cannot receive feedback signals of "work completed" (i.e., negative feedback is weakened). It will continue to secrete FSH excessively, attempting to "urge" the ovaries to work. Therefore, a significant increase in FSH levels is a core indicator for diagnosing premature ovarian failure.
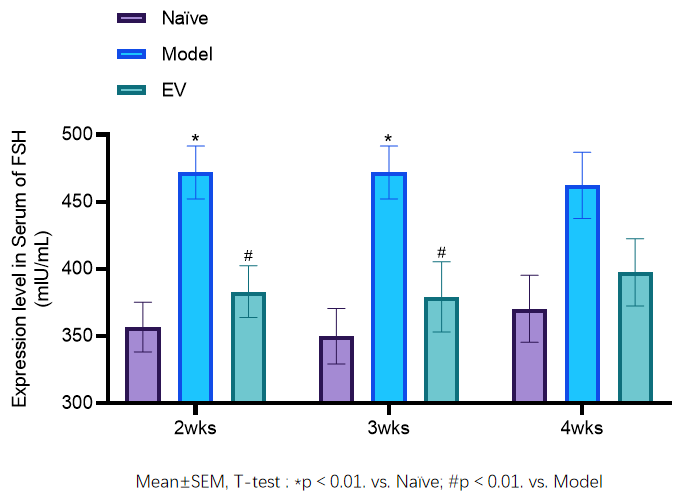
• In the POF rat model, a decrease in follicle count and a decrease in estrogen (E2) levels can cause compensatory elevation of follicle stimulating hormone (FSH) and luteinizing hormone (LH) secreted by the pituitary gland.
• The model control group showed a continuous increase in FSH levels within 4 weeks, with FSH levels significantly higher than baseline in the second week (p<0.05). After treatment, the EV group had lower FSH levels than the model group in the second, third, and fourth weeks, and FSH levels significantly decreased in the second week (p<0.01 vs model group). Estrogen supplementation had a certain therapeutic effect.
Changes in serum luteinizing hormone (LH) levels
LH (Luteinizing Hormone)
• Source: Also secreted by the pituitary gland.
• Function: Based on the action of FSH, LH triggers ovulation (causing mature follicles to rupture) and promotes the transformation of ovulated follicles into corpus luteum. The corpus luteum is responsible for secreting progesterone and estrogen.• Meaning in POI model: Similar to FSH, due to the reduced responsiveness of the ovaries to gonadotropins, the brain continues to secrete large amounts of LH in an attempt to stimulate ovulation and corpus luteum formation. Therefore, LH levels usually also increase.
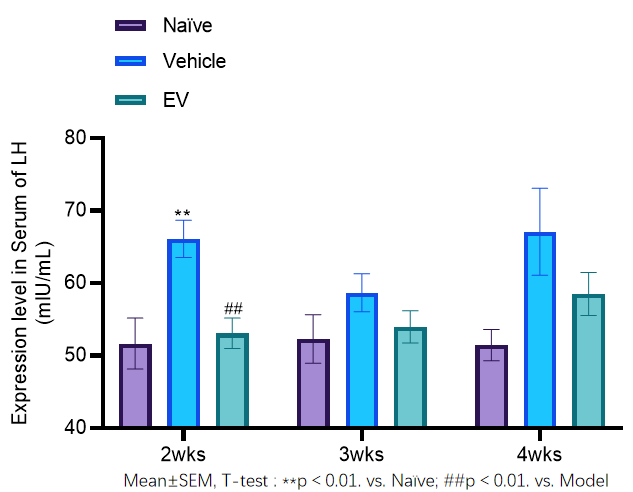
The LH levels in the model control group continued to increase within 4 weeks, with LH levels significantly higher than baseline in week 2 (p<0.01). After treatment, the LH levels in the EV group were lower than those in the model group in weeks 2, 3, and 4, and decreased significantly in week 2 (p<0.01 vs model group).
Ovarian index
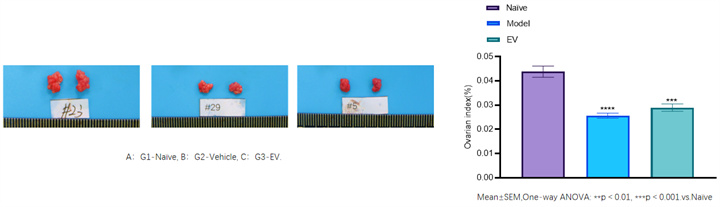
• The ovarian index (ovarian weight/body weight) reflects the relative quality of the ovaries.
• Modeling resulted in significantly lower ovarian index in the model group compared to the normal group (ovarian atrophy, p<0.05); 0.0001). The ovarian index of the treatment group was still lower than normal levels (due to irreversible ovarian damage), but there was some improvement compared to the model group.
Histopathological evaluation (HE staining)
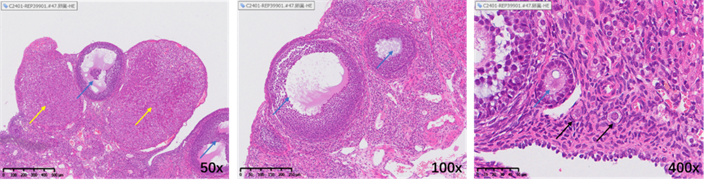
Illustration: The yellow arrow indicates the corpus luteum, the blue arrow indicates the growing follicle, and the black arrow indicates the primordial follicle.
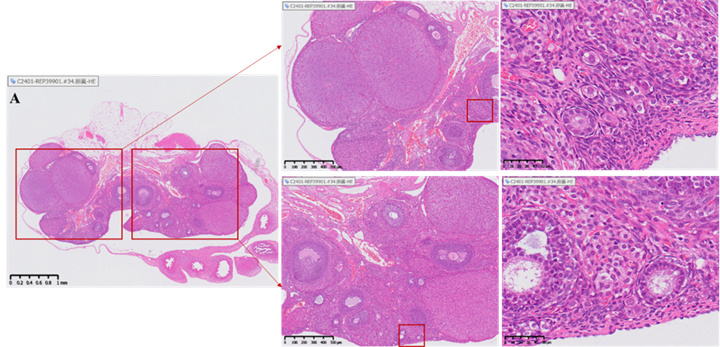
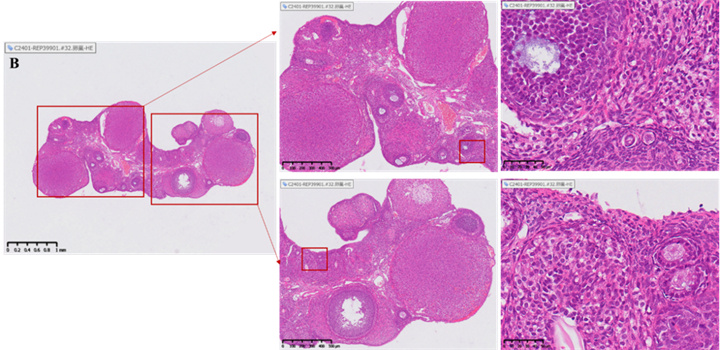
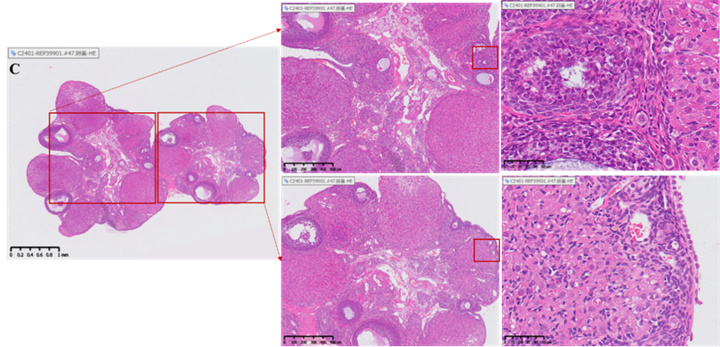
Changes in the number of follicles and corpus luteum in the ovary
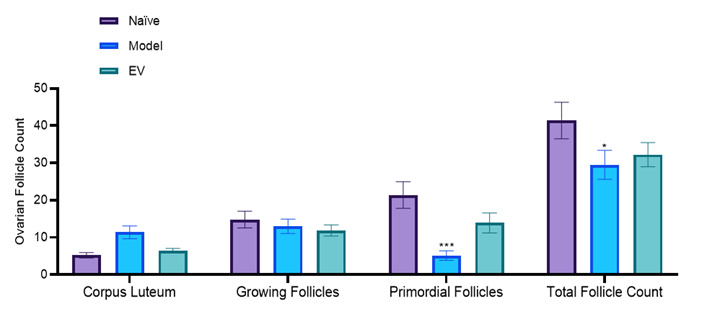
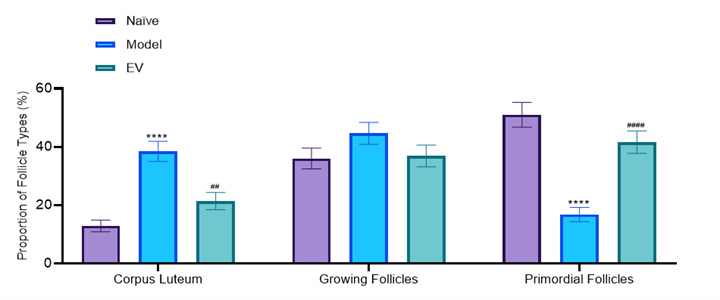
Two-way ANOVA: *p<0.05,***p<0.001. vs. Naïve;
##p<0.01, ####p<0.0001. vs. Model
• Healthy ovaries contain abundant reserves of primordial follicles.
• The ovarian slices of the model group rats showed depletion of follicle reserve: the total number of follicles and primordial follicles in the ovaries were significantly lower than those in the normal control group (total follicles ↓, p<0.05); 0.05; Primordial follicle ↓, p< 0.001), while the number and proportion of corpus luteum significantly increased (p<0.0001), reflecting premature ovarian emptying.
• After treatment, follicular development was reduced to a certain extent: the EV group reduced the proportion of corpus luteum and increased the proportion of primordial follicles, but the improvement in total follicle count was limited.
• Summary: Estrogen replacement therapy can improve hormone imbalance and ovarian histological damage caused by POF to a certain extent.
05.Summary and Discussion
Comparative analysis of therapeutic efficacy with positive control EV
• Introduction to Positive Control EV: Estradiol valerate (EV) is a commonly used estrogen replacement therapy used to replace endogenous estrogen, alleviate POF symptoms, and partially restore ovarian axis hormone balance.
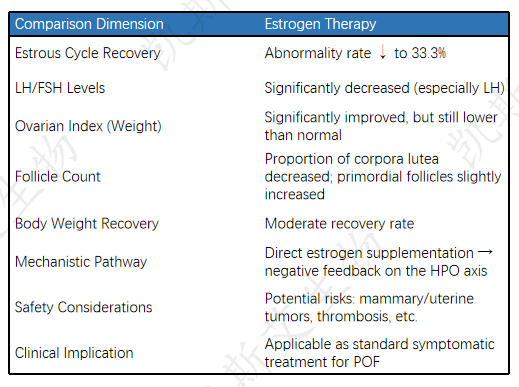
Summary and Clinical Implications
Model effectiveness: The cyclophosphamide induced POF model in SD rats successfully reproduced key features of premature ovarian failure (hormone changes, ovarian atrophy, follicle reduction, etc.), providing reliable evidence for preclinical evaluation of new therapies. The expected effect of the positive control EV in the model validated the clinical relevance of the model.




