1、 Introduction to influenza virus
Influenza is an acute respiratory infectious disease caused by influenza virus, which is highly contagious. According to the World Health Organization (WHO), there are approximately 3-5 million severe cases and 290000-650000 deaths worldwide each year due to influenza related complications. Since records began, there have been several influenza pandemics in human history, resulting in over 100 million deaths.
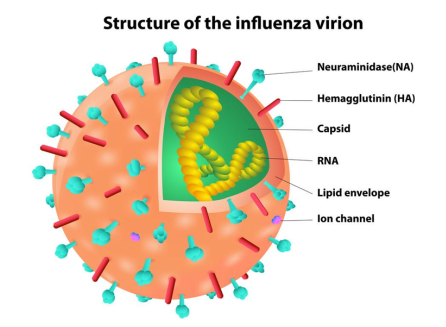
The influenza virus belongs to the family Orthomyxoviridae and is divided into four types: A, B, C, and D. Among them, A and B are the main pathogens that cause seasonal influenza in humans. The hemagglutinin (HA) and neuraminidase (NA) proteins of type A influenza virus are prone to mutation, resulting in the formation of new subtypes (such as H1N1, H3N2, H5N1, etc.). This mutation is mainly achieved through two ways: antigen drift (small mutations leading to annual changes in the epidemic strain) and antigen transformation (recombination of different subtypes of genes, which may trigger a pandemic). For example, the 2009 H1N1 pandemic was caused by a new strain of influenza virus produced by the recombination of swine influenza, avian influenza, and human influenza viruses.
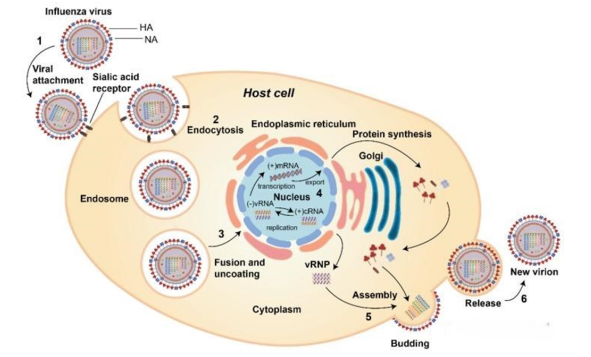
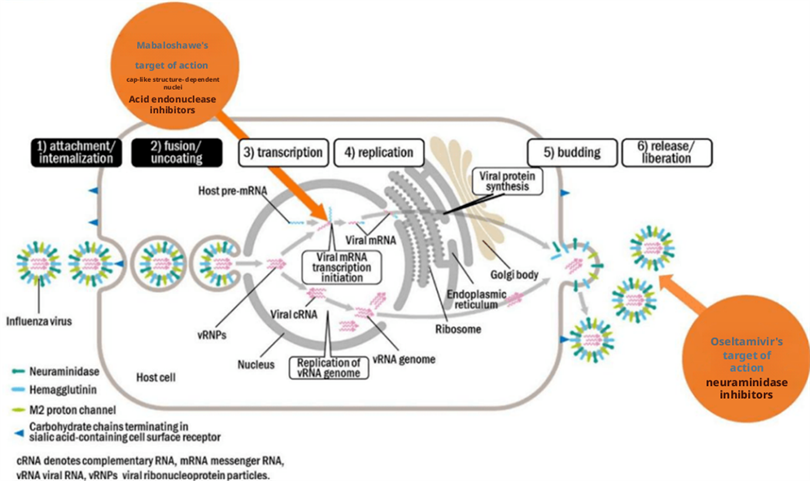
The main replication site of influenza virus in the human body is in the respiratory epithelial cells. The replication cycle is that HA binds to sialic acid receptors on the host cell membrane and is transported to the cytoplasm through endocytosis. Then, with changes in environmental pH, the conformation of viral HA changes, inducing fusion between the viral outer membrane and the cell membrane. The result of membrane fusion is the release of the genome encapsulated within the virus into the cytoplasm, which is then transported to the nucleus. The viral genome replicates itself in the cell nucleus and transcribes mRNA. The viral mRNA is then transferred to the cytoplasm to translate viral proteins. Afterwards, the viral genome and proteins are transported to the vicinity of the cell membrane for assembly, forming a new virus. During the release process of a new virus on the cell membrane, the NA on the surface of the virus cuts off the binding between the HA on the surface of the new virus particles and the sialic acid on the cell membrane, causing the virus to detach from the cell and be released outside the cell.
2、 Introduction to the current status of research and development of
influenza vaccines or therapeutic drugs
01.Current status of influenza vaccine research and development
Influenza vaccine is one of the main measures for preventing and controlling influenza. It can reduce the chances of vaccination recipients contracting influenza or alleviate flu symptoms. The influenza vaccines that have been launched globally include inactivated vaccines (including split vaccines and subunit vaccines), attenuated live vaccines, and recombinant vaccines. According to the components contained in the vaccine, it can be divided into trivalent and quadrivalent influenza vaccines. Compared with trivalent influenza vaccines, quadrivalent influenza vaccines add a lineage of influenza B to the strain. Inactivated vaccine was injected intramuscularly, and attenuated live vaccine was given by intranasal spray. However, due to the rapid mutation of influenza virus, the epidemic strain and vaccine strain may not be consistent, resulting in differences in the effectiveness of influenza vaccines; In addition, using chicken embryos to produce seasonal influenza vaccines has the problem of slow production speed, and when adaptive mutations occur in chicken embryos, the effectiveness of the vaccine will be reduced. More importantly, the emerging influenza pandemic will render seasonal influenza vaccines almost ineffective, and the general lack of immunity in the population will exacerbate symptoms. Therefore, it is urgent to develop an effective universal influenza vaccine.
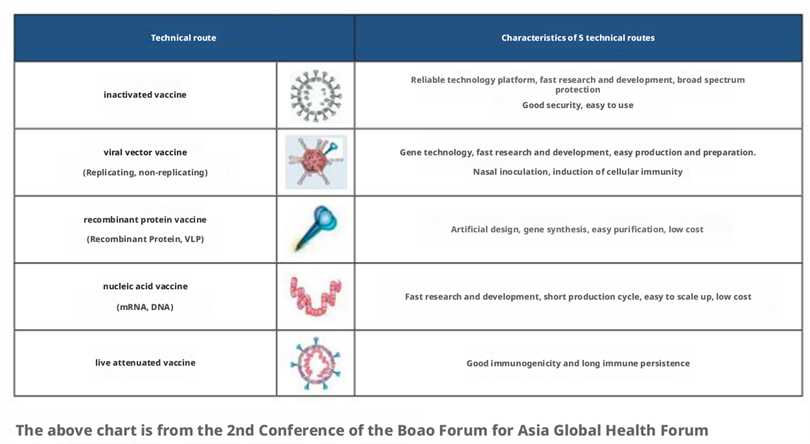
Figure 3: Current Status of Influenza Vaccine Development
The replication of influenza virus relies on several key proteins within it, such as PA, PB1, PB2 (which together form the RNA polymerase complex), and neuraminidase (NA). Most existing drugs attack these targets.
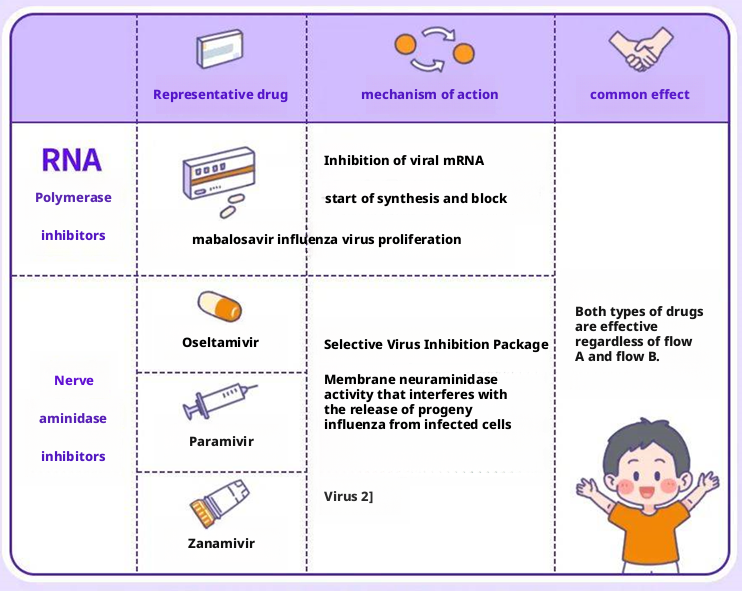
Figure 4: Introduction to influenza therapeutic drugs
3、 Application of animal models in preclinical development of influenza vaccines and drugs
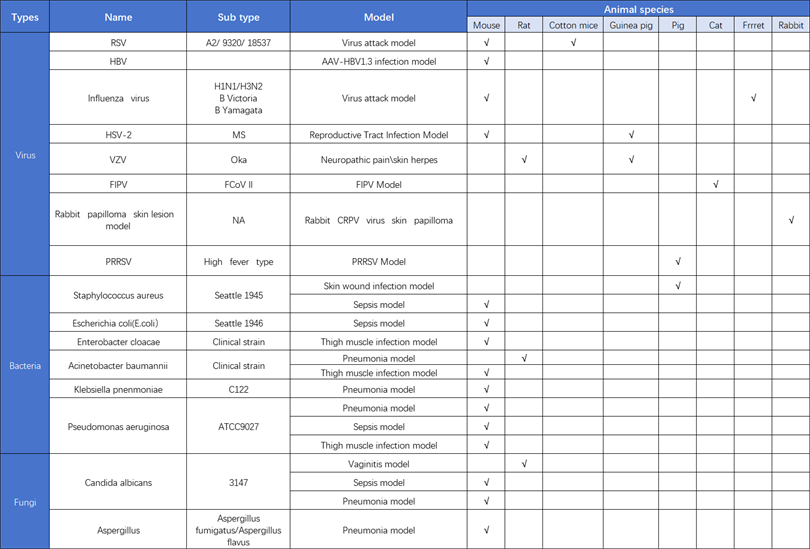
The most widely used animal model for influenza virus currently is the mouse infection model. The mouse model is easy to operate and manage, and can use inbred mice to reduce individual differences, which is easier to control than other animal species and relatively cheaper in price. However, mice are not natural hosts of influenza, so they cannot simulate the symptoms and related pathology of human influenza infection well, and there are significant differences in immune responses between mice and humans. Therefore, the mouse infection model is more suitable for the early stages of vaccine and drug screening.
The second widely used animal model is the ferret infection model. Compared to mice, the physiological structure and anatomy of the respiratory tract of ferrets are closer to those of humans. The clinical symptoms of influenza virus infection are also very similar to those of human influenza, including coughing, runny nose, sneezing, and fever. Therefore, the ferret infection model serves as the "gold standard model" for the preclinical effectiveness evaluation of influenza vaccines and drugs
4、 KCI BioTech Anti infective Vaccine and Drug Development Service Platform
KCI • KMQ has established an anti infection research and development service platform that has dual qualifications of BSL-2 and ABSL-2, covering over 100 pathogenic microorganisms, including bacteria, viruses, and fungi required for human drug development research, veterinary medicine, and pet medicine. The BSL-2 laboratory covers an area of approximately 200m ² and contains cells, viruses, and bacteria; The experimental area of ABSL-2 is about 1000m ², including 300m ² for small animal laboratory and 700m ² for large animal laboratory. After years of internal research and development and external services, KCI • KMQ has successfully established over 20 animal models of viral, bacterial, and fungal infections, providing customers with high-quality pharmacological and pharmacodynamic evaluation services.
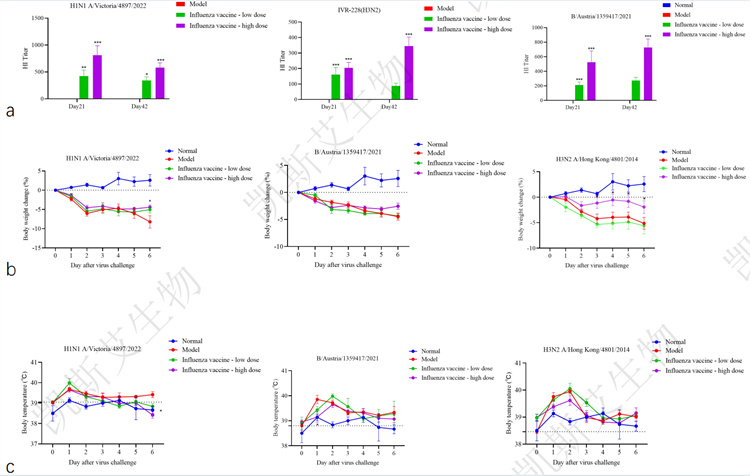
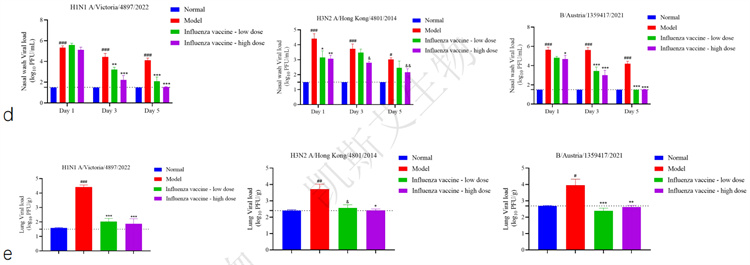
01. Evaluation of immunogenicity and antiviral protection of influenza vaccine in ferrets
data display
▪ a-hemagglutination inhibition potency detection, b-animal weight changes,
c-animal body temperature changes, d-nasal wash virus load, e-pulmonary virus load
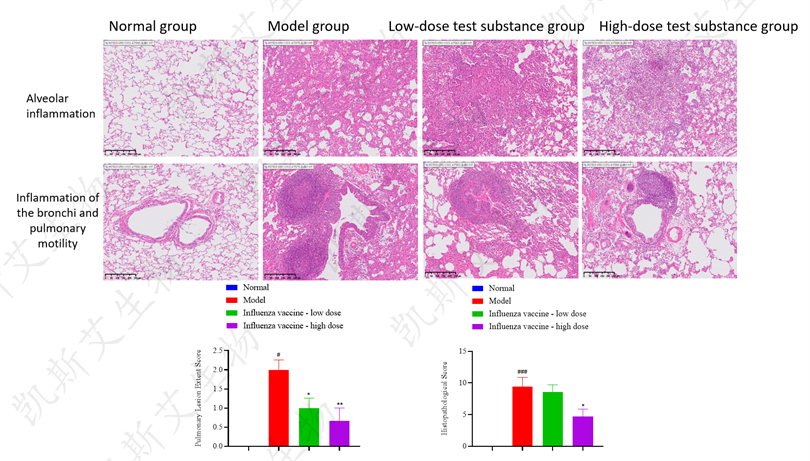
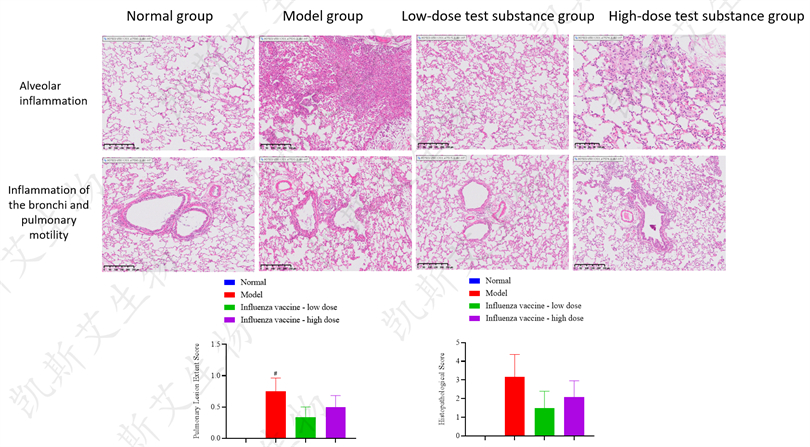
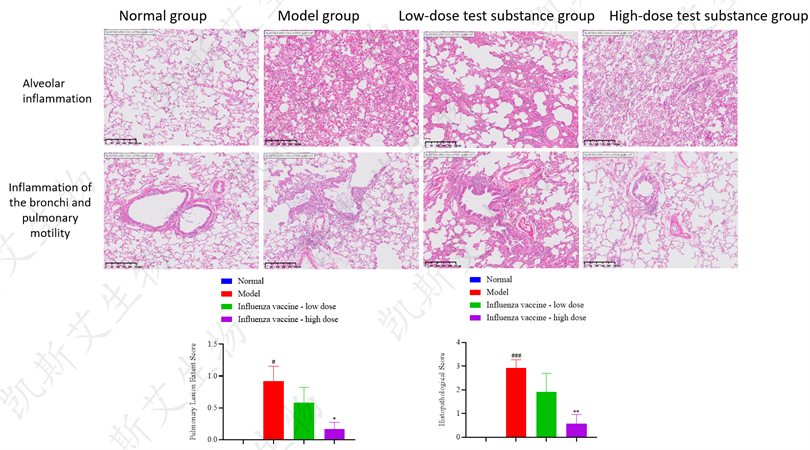
▪ HE Pathological Staining Analysis of Lungs - HNN1 Attack, H3N2 Attack, BV Attack
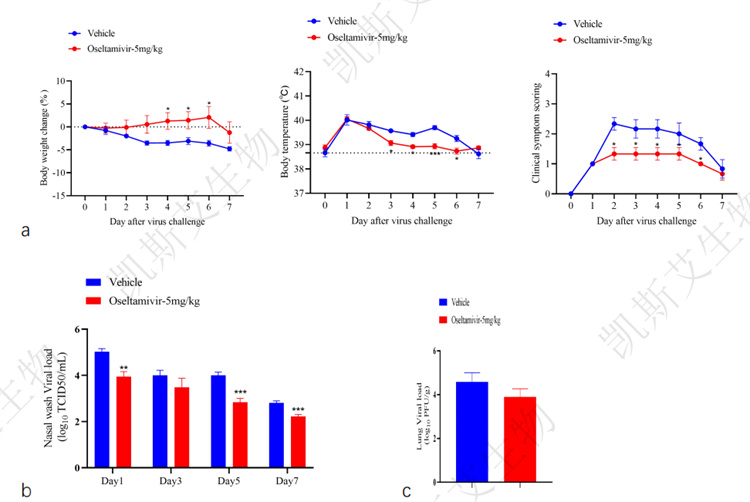
02.Study on the antiviral effect of oseltamivir in ferret influenza virus infection model
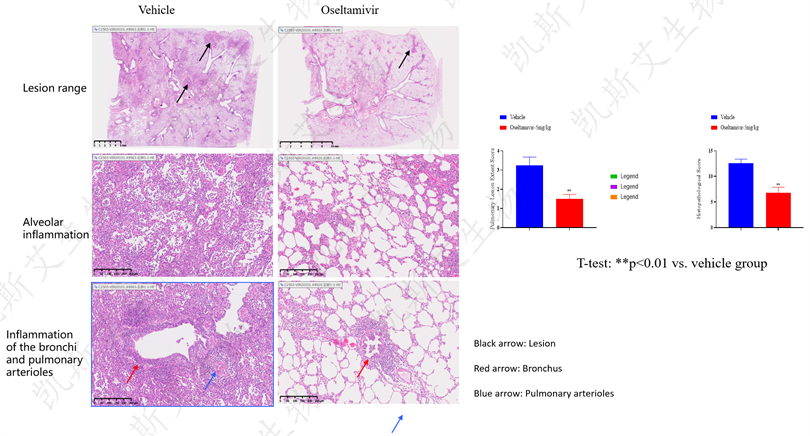
data display▪ a-Animal weight/body temperature/clinical symptom score, b-Nasal wash fluid virus load detection,
c-Lung load detection
▪ Pathological staining analysis of lung HE